VAXANIX PIPELINE
Vaxanix offers a risk-diversified pipeline spanning a multitude of clinical stages, unique mechanisms of action, regulatory pathways, geographies, and technology platforms. Our pipeline includes candidates for solid and liquid tumors, as well as autoimmune, inflammatory, and fibrotic conditions. Our products offer first-in-class or best-in-class potential, with our platforms providing innovative solutions for the next 20+ years.
Our Pipeline achievements:
- Commercial ready product (European approval)
- Phase 3 candidate
- FDA Breakthrough Device Designation
- Three Orphan Disease Designations
- Rare Pediatric Priority Review Designation, with the potential for 2 more
- 50+ global patents
Pipeline Charts
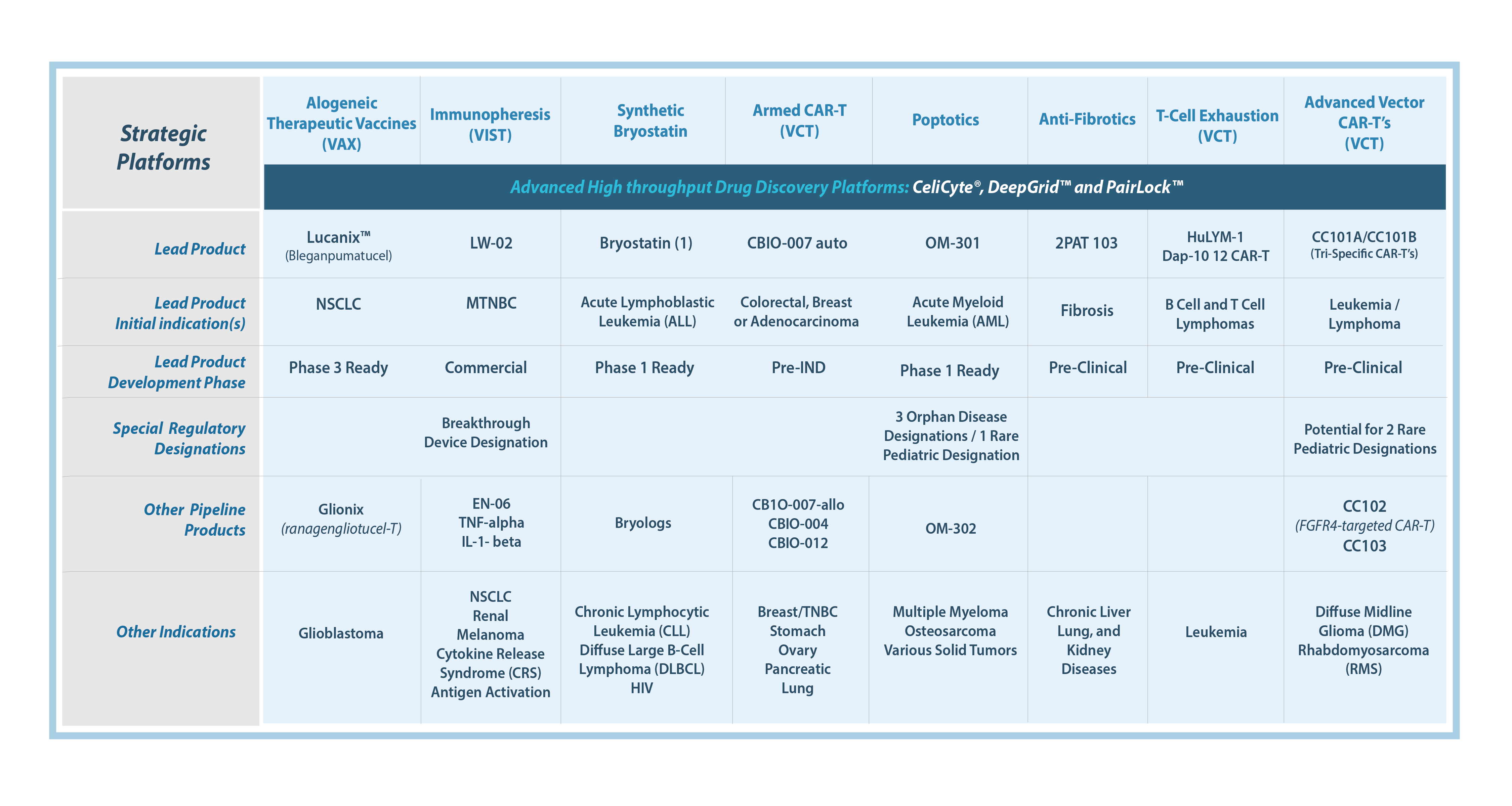
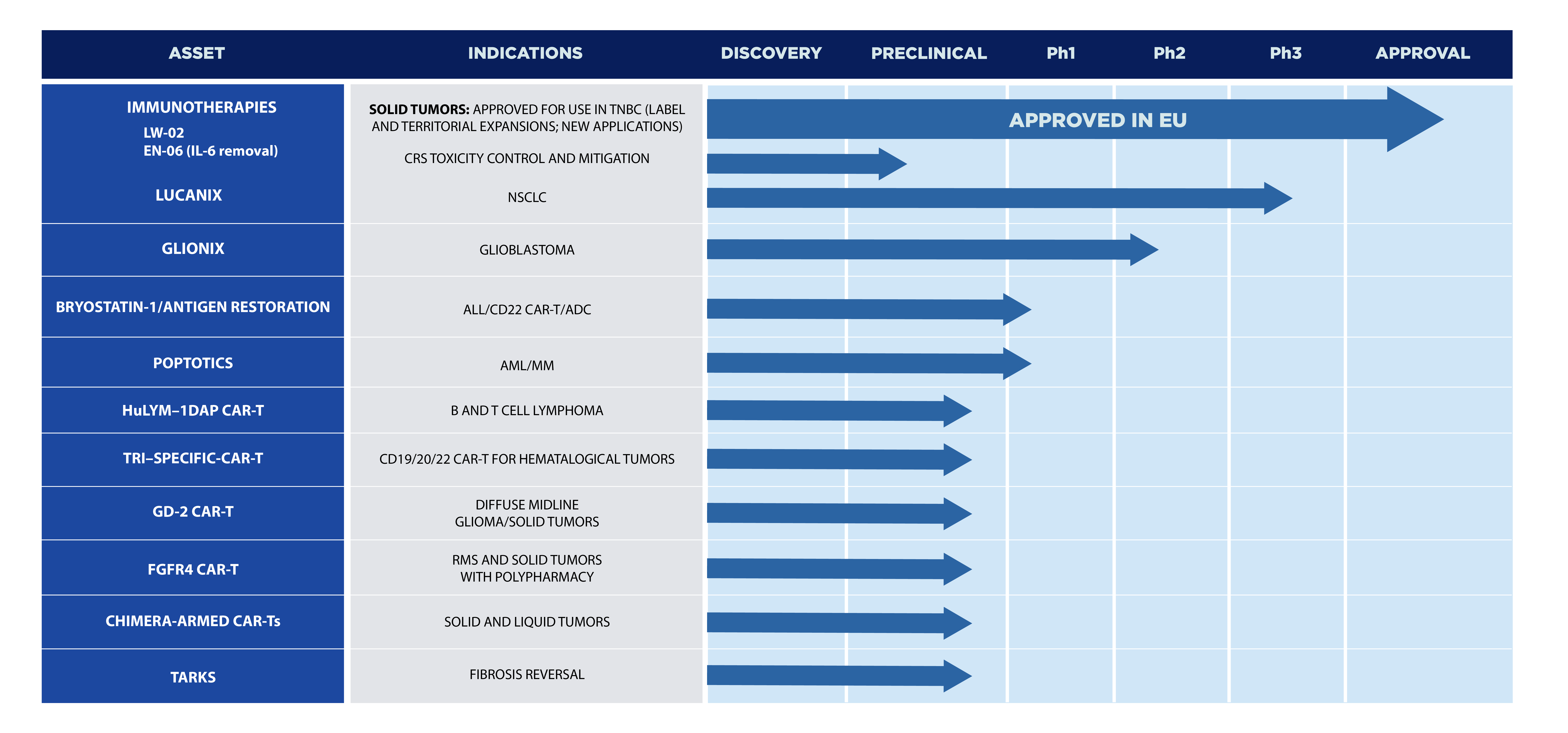
* The LW-02 Column is indicated for use with the Spectra Optia® Apheresis System (Terumo BCT, Inc.) to remove soluble Tumor Necrosis Factor Receptors (sTNF-Rs) 1 and 2 from the plasma of patients ages 18 and older with advanced, refractory, triple-negative breast cancer. However, the clinical efficacy of the LW-02 Column has not yet been demonstrated. Clinical investigations evaluating the clinical efficacy of the LW-02 Column for this indication are ongoing.
