Creating durable next generation imuno-oncology products
Lucanix
a novel, best in class, late-stage cancer vaccine for nonsmall cell lung cancer (NSCLC).
Glionix
a version of Lucanix customized to treat glioblastoma (brain cancer) to be developed in collaboration with the Peter MacCallum Cancer Institute.
HuLym-1
novel CAR-T construct to address both hematological malignancies and solid tumors.
Therapeutics
A pipeline of candidate therapeutics designed with “Antigen Escape” in mind and other attributes.

Technologies
Cutting-edge immuno-oncology
technologies & therapies addressing
“Antigen Escape”
- Immuno-oncological approaches to treating cancer have made tremendous strides in recent years.
- Checkpoint inhibitors, CAR-Ts, bi-specific antibodies and other treatments have improved the outcome for many cancer patients.
- Nevertheless, relapse not long after treatment remains an obstacle, in many cases due to a phenomenon known as “antigen escape” or “immunoediting”.
- Over time, many cancer antigen targets succumb to pressure from the targeting therapeutic and down regulate expression on the surface of the cancer cell, leading to relatively early relapse rates.
- This is true for both solid tumors and hematological tumors, and necessitates deployment of a variety of solutions to address the problem and provide patients with the duration of benefit they deserve.
Developing next-generation multi-neoantigen vaccines with the ability to address multiple cancer targets, complemented by a portfolio of novel CAR-T therapeutics
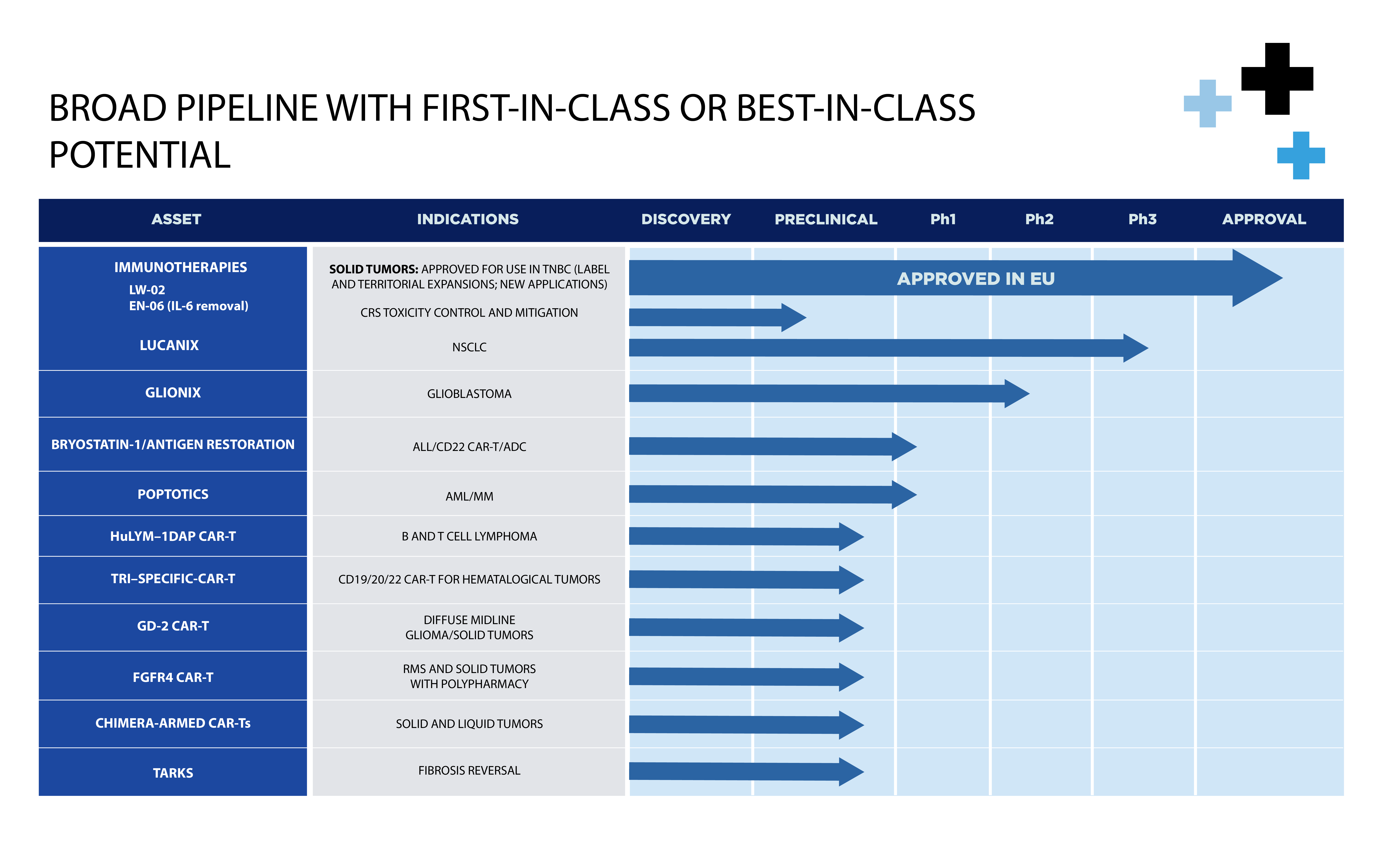
Diversified pipeline of potential “best in class” assets for solid and hematological tumors:
LUCANIX
a novel, best in class, late-stage multi-neoantigen cancer vaccine for non-small cell lung cancer (NSCLC) with highly statistically significant efficacy data in advanced clinical trials, and
GLIONIX
a version of Lucanix customized to treat glioblastoma (brain cancer) to be developed in collaboration with the Peter MacCallum Cancer Institute, the leading cancer center in Australia.
HuLYM-1 DAP CAR-T
novel CAR-T construct to address both hematological malignancies and solid tumors.
TRISPECIFIC duoCAR-T
targeting CD19, CD20 and CD22 for B cell tumors.
GD2 CAR-T
for mutated diffuse midline gliomas, a rare pediatric glioma, other CNS tumors and certain sarcomas.
FGFR4 CAR-T
to treat RMS, a soft tissue cancer in children, and various solid tumors
Lead candidate, Lucanix, is clinically de-risked with highly statistically significant data demonstrating B and T cell response:
- Phase 2 and Phase 3 data demonstrating improved survival in late stage NSCLC patients
- Minimal adverse events and toxicity
- Off-the-shelf therapy and highly efficient manufacturing process allows for attractive margins
- Single, monthly intradermal injections for ease of use
- Potentially synergistic with other therapies
Significant [$5B+] market potential addressing diseases with significant unmet need including NSCLC, glioblastoma, and B and T cell lymphoma
Highly experienced management team and renowned advisory board with deep expertise in developing innovative technologies
~$106M invested in technology to date
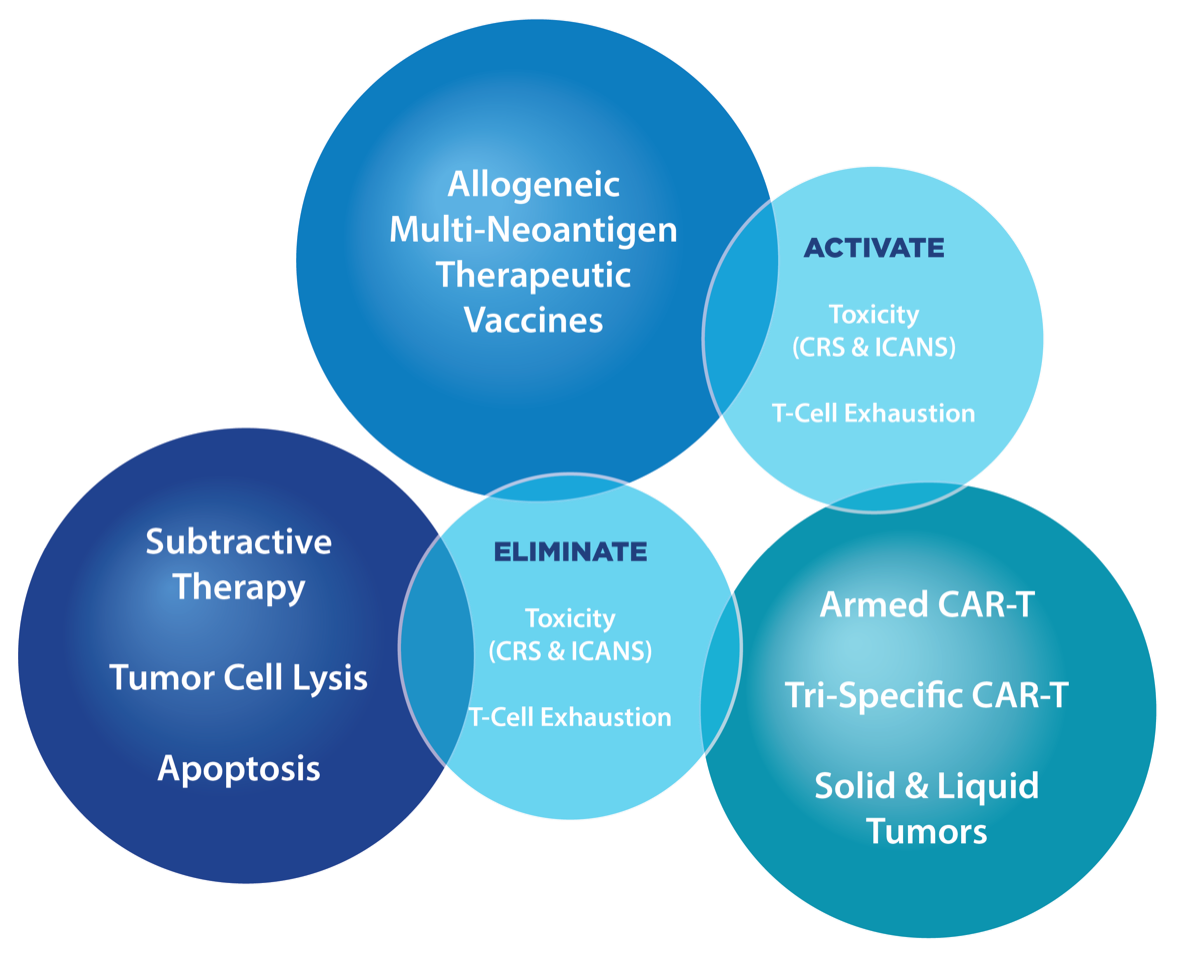
An industry-first pipeline of platforms and products which provides next generation therapeutic approaches to both solid and liquid tumors. These work in conjunction with solutions designed to enhance therapeutic response and durability whilst minimizing toxicity, CRS, ICANS, and T-Cell exhaustion.
- Immuno-oncological approaches to treating cancer have made tremendous strides in recent years.
- Checkpoint inhibitors, CAR-Ts, bi-specific antibodies and other treatments have improved the outcome for many cancer patients.
- Nevertheless, relapse not long after treatment remains an obstacle, in many cases due to a phenomenon known as “antigen escape” or “immunoediting”.
- Over time, many cancer antigen targets succumb to pressure from the targeting therapeutic and down regulate expression on the surface of the cancer cell, leading to relatively early relapse rates.
- This is true for both solid tumors and hematological tumors, and necessitates deployment of a variety of solutions to address the problem and provide patients with the duration of benefit they deserve.
Developing next-generation multi-neoantigen vaccines with the ability to address multiple cancer targets, complemented by a portfolio of novel CAR-T therapeutics
Diversified pipeline of potential “best in class” assets for solid and hematological tumors:
LUCANIX
a novel, best in class, late-stage multi-neoantigen cancer vaccine for non-small cell lung cancer (NSCLC) with highly statistically significant efficacy data in advanced clinical trials, and
GLIONIX
a version of Lucanix customized to treat glioblastoma (brain cancer) to be developed in collaboration with the Peter MacCallum Cancer Institute, the leading cancer center in Australia.
HuLYM-1 DAP CAR-T
novel CAR-T construct to address both hematological malignancies and solid tumors.
TRISPECIFIC duoCAR-T
targeting CD19, CD20 and CD22 for B cell tumors.
GD2 CAR-T
for mutated diffuse midline gliomas, a rare pediatric glioma, other CNS tumors and certain sarcomas.
FGFR4 CAR-T
to treat RMS, a soft tissue cancer in children, and various solid tumors
Lead candidate, Lucanix, is clinically de-risked with highly statistically significant data demonstrating B and T cell response:
- Phase 2 and Phase 3 data demonstrating improved survival in late stage NSCLC patients
- Minimal adverse events and toxicity
- Off-the-shelf therapy and highly efficient manufacturing process allows for attractive margins
- Single, monthly intradermal injections for ease of use
- Potentially synergistic with other therapies
Significant [$5B+] market potential addressing diseases with significant unmet need including NSCLC, glioblastoma, and B and T cell lymphoma
Highly experienced management team and renowned advisory board with deep expertise in developing innovative technologies
~$106M invested in technology to date
Vaxanix by the numbers: Pipeline Highlights
1
EU-MDR approved TNBC product; with label expansions in process. FDA Breakthrough Designation.
1
Product candidate with compelling Phase 3 data for NSCLC
1/3/1
3 Orphan Disease Designations
1 Rare Pediatric Designation
(with potential for 2 more)
12+
Pipeline Programs
7
Core technologies with multiple product opportunities and complementary modalities
We offer a risk-diverse pipeline spanning a multitude of clinical stages, mechanisms of action, regulatory pathways, geographies, and technology platforms. Our technologies have been granted over 25+ patents worldwide and have an additional 50 patent applications in process.
Vaxanix and its subsidiaries have thus far raised over $200 million to advance R&D, development, commercialization, and expansion of discovery programs and platforms.
Vaxanix Platform Technologies
VIST (Vaxanix Immunopheresis® Subtractive Therapy)
VIST represents a new therapeutic approach. VIST is designed to subtract or selectively remove targeted molecules, such as cytokines, proteins, and soluble receptors, to stabilize the immune system, activate antigens, and reduce toxicity. Our first VIST product, LW-02 Column, is commercially available in Europe.
VIST (Vaxanix Immunopheresis® Subtractive Therapy
VIST represents a novel therapeutic approach for immune modulation. VIST is designed to subtract or selectively remove targeted molecules, such as cytokines, proteins, and soluble receptors, to stabilize the immune system, activate antigens, convert tumors from cold to hot, remove proteins and other factors causing resistance or limited durability, and reduce toxicity. VIST can be designed to target any soluble factor and neutralize its effect outside of the body. VIST products are designed to be used safely with all existing therapeutic products. Our first VIST product, LW-02 Column, is commercially available in Europe and has garnered FDA Breakthrough Device Designation in the US; our second product, EN-06, is in preclinical testing.
VIST allows products to be developed for target capture through proprietary peptides of virtually any specific soluble factor (cytokines, interleukins, galectins, growth factors), utilizing Vaxanix’ apheresis column technology connected to existing apheresis/plasmapheresis machines. This approach does not administer a drug or chemical into the patient to achieve the desired results. Instead, by lowering the concentration of the offending target molecule, we get safe immune modulation without toxicity, quality of life compromises or traditional side effects. VIST products can work alone, in combination with other standards of care or as an adjunctive therapy to treat solid and liquid tumors, auto-immune diseases, and inflammatory diseases.
LW-02, our first product under the VIST platform, has received CE Mark approval in Europe and FDA Breakthrough Device Designation. It is designed for all solid tumors and targets the safe removal of sTNF-Rs 1&2. LW-02 is available in select European countries through our distribution partner. LW-02 has shown promising results in cancers such as TNBC, Breast, NSCLC, melanoma, renal carcinoma, as well as the reduction of metastatic tumors. It has demonstrated antigen activation in the tumor microenvironment as well as potentially converting cold tumors to hot tumors in refractory patients. LW-02 is designed to be used safely with existing cancer therapeutics.
EN-06 is being developed to target the safe removal of IL-6 without using antibodies or immunosuppressive agents. Our EN-06 column based on the VIST platform is designed to be safe, non-toxic, and cost-effective, which may allow prophylactic administration to all CAR-T patients, thereby potentially mitigating the risk of CRS and ICANS. If successful, EN-06 Column could significantly expand the CAR-T market by allowing patients to safely receive and complete their full courses of treatment.
VAV™ (Vaxanix Allogeneic Therapeutic Vaccines)
Allogeneic therapeutic anticancer vaccines have been pursued due to their high likelihood of success and an expected low level of side effects and safety issues. Our first VAV targeting NSCLC has exhibited positive results in early Phase III trials. We also have potentially positive early development data for a GBM vaccine.
VAV™ (Vaxanix Allogeneic Therapeutic Vaccines)
Allogeneic therapeutic anticancer vaccines have been pursued due to their high likelihood of success and an expected low level of side effects and safety issues. Our first VAV targeting non-small cell lung cancer (NSCLC) has exhibited positive results in Phase III trials. We also have potentially positive early development data for a glioblastoma (GBM) vaccine.
Vaxanix VAV platform allows the development of multi-epitope allogeneic therapeutic vaccine products that address all known epitopes for the targeted cancer. Allogeneic therapeutic vaccines have been pursued due to their high likelihood of success and an expected low level of side effects and safety issues. As off the shelf products, allogeneic vaccines generally have a lower cost of goods vs. those produced with autologous cells.
Our lead product, Lucanix®, can be delivered intradermally on a monthly basis. It has shown highly statistically significant data in Phase 3 US trials for late-stage NSCLC, including squamous cell lung cancer, with minimal adverse events and toxicity.
VCT™ (Vaxanix Cellular Therapeutics)
Our VCT platform represents a highly versatile CAR-T platform that offers Tri-specific CAR-T therapies as well as cell therapies designed to selectively seek out targets, and cell types, to deliver virtually any therapeutic payload while minimizing T-Cell exhaustion.
VCT™ (Vaxanix Cellular Therapeutics)
Our VCT platform represents a highly versatile CAR-T platform that offers Tri-specific CAR-T therapies as well as cell therapies designed to selectively seek out targets, and cell types to deliver virtually any therapeutic payload while minimizing T-Cell exhaustion.
VCT also offers a novel Tri-specific CAR-T approach using state of the art lentiviral vectors and is designed to avoid resistance. The third element of our VCT platform exploits the stable LYM target (HuLym-1) and is designed to minimize T-cell exhaustion and reduction of off-target effects.
The VCT platform is a unified constellation of next-generation, highly-selective cellular therapeutic technologies designed for solid and liquid tumors, with lower toxicity and improved safety profiles. The platform utilizes a novel CAR-T construct that can be armed with any immunomodulatory payload being delivered in a highly targeted manner to the tumor micro-environment, providing the possibility of treating solid and liquid tumors with lower toxicity.
Synthetic Bryostatin
Current Bryostatin products based on natural sources cost upwards of $20M per gram. Our synthetic Bryostatin molecule, Bryostatin-1, is orders of magnitude less expensive, providing a viable use of these molecules. Our leading molecule is initially targeting Acute Lymphoblastic Leukemia (ALL). Bryostatin-1 offers increased target antigen expression making tumor cells more “visible”, resulting in increased immuno-oncology agent kill rate effectiveness. It also offers the potential to increase response and reduce relapse rates for patients treated with CAR-T, Antibody Drug Conjugates (ADC), and Monoclonal Antibodies (mAB) agents.
Synthetic Bryostatin
Current Bryostatin products based on natural sources cost upwards of $20M per gram. Our synthetic Bryostatin molecule, Bryostatin-1, is orders of magnitude less expensive, providing a viable use of these molecules. Our leading molecule is initially targeting Acute Lymphoblastic Leukemia (ALL). Bryostatin-1 offers increased target antigen expression making tumor cells more “visible”, resulting in increased immuno-oncology agent kill rate effectiveness. It also offers the potential to increase response and reduce relapse rates for patients treated with CAR-T, Antibody Drug Conjugates (ADC), and Monoclonal Antibodies (mAB) agents.
Poptotics
Our groundbreaking novel technology specifically targets cancer cells and cancer stem cells, while sparing normal cells. The mechanism of action is to poke holes in the cancer cell membrane, killing the cancer cells and, at the same time, stimulating the patient’s own immune system to fight the cancer.
Poptotics
Our groundbreaking novel technology specifically targets cancer cells and cancer stem cells, while sparing normal cells. The mechanism of action is to poke holes in the cancer cell membrane, killing the cancer cells and, at the same time, stimulating the patient’s own immune system to fight the cancer.
The Poptotics technology takes advantage of the presence of a unique cell surface protein target found only on the surface of cancer cells and cancer stem cells. The lead drug candidate, OM-301, binds to the cancer cell surface and then causes pores (holes) to form in the cancer cell membrane. These pores allow fluid to rush into the cancer cell, which then expands like a balloon and bursts (pops). The bursting immediately kills the cancer cell while exposing previously hidden cancer cell antigens to the patient’s immune system, and is expected to have a secondary action as a personalized vaccine against the cancer.
The target has been identified on many different types of cancer cells, both solid cancers and hematologic malignancies, giving the potential for a huge addressable market. The target does not exist on normal cells, and preclinical animal data has shown no off-target toxicity. The fact that the target has also been identified on some types of cancer stem cells is an exciting finding that augurs well for potentially curative treatment.
Poptotics has several advantages over oncolytic viruses (which also cause cancer cell bursting, but from the inside, not the outside). First, OM-301 is inexpensive to manufacture, and the target is present on multiple cancers so no individualized modification of the drug is necessary. In contrast, oncolytic viruses require complex manufacturing and stabilization, and require viral modification for different tumor types. Second, the immune response does not decrease the effectiveness of the drug, but rather enhances is. The immune response against oncolytic viruses can decrease their effectiveness by neutralizing the virus; therefore, repeat dosing may be limited. Third, Poptotic drugs attack an identified druggable target, whereas dosing (accurate titration) of oncolytic viruses can be a problem.
Vaxanix Therapeutics Development Platform
Through its DeepGrid™ and SingleCyte® Technologies, Vaxanix profiles human immunologic cells, proteins and pathways at unprecedented scale and speed, shrinking new drug discovery timelines from years to days.
Vaxanix Therapeutics Development Platform
Through its DeepGrid™ and SingleCyte® Technologies, Vaxanix profiles human immunologic cells, proteins and pathways at unprecedented scale and speed, shrinking new drug discovery timelines from years to days.Anti-Fibrotics TARK
Our unique anti-fibrotic platform uses the conserved ‘K1 loop’ portion of hepatocyte growth factor (HPC) to synthesize organ-specific molecules that can overcome the pro-fibrotic effects of transforming growth factor beta-1 (TGF-β1). Currently in preclinical testing, Vaxanix’ TARK platform offers potential treatments for numerous chronic fibrotic diseases involving the liver, kidneys, lungs, intestines and eyes and other organs
Anti-Fibrotics TARK
Our unique anti-fibrotic platform uses the conserved ‘K1 loop’ portion of hepatocyte growth factor (HPC) to synthesize organ-specific molecules that can overcome the pro-fibrotic effects of transforming growth factor beta-1 (TGF-β1). Currently, in preclinical testing, Vaxanix’ TARK platform offers potential treatments for numerous chronic fibrotic diseases involving the liver, kidneys, lungs, intestines and eyes and other organs.Vaxanix Platform Technologies